Mildred Dresselhaus’ life was one in defiance of odds. Growing up poor in the Bronx — and even more to her detriment, growing up a woman in the 1940s — Dresselhaus’ traditional career options were paltry. Instead, she rose to become one of the world’s preeminent experts in carbon science as well as the first female Institute Professor at MIT, where she spent 57 years of her career. She collaborated with physics luminaries like Enrico Fermi and laid the essential groundwork for future Nobel Prize winning research, directed the Office of Science at the U.S. Department of Energy and was herself awarded the National Medal of Science.
In the excerpt below from Carbon Queen: The Remarkable Life of Nanoscience Pioneer Mildred Dresselhaus, author and Deputy Editorial Director at MIT News, Maia Weinstock, tells of the time that Dresselhaus collaborated with Iranian American physicist Ali Javan to investigate exactly how charge carriers — ie electrons — move about within a graphite matrix, research that would completely overturn the field’s understanding of how these subatomic particles operate.

MIT Press
Excerpted from Carbon Queen: The Remarkable Life of Nanoscience Pioneer Mildred Dresselhaus by Maia Weinstock. Reprinted with permission from The MIT Press. Copyright 2022.
A CRITICAL ABOUT-FACE
For anyone with a research career as long and as accomplished as that of Mildred S. Dresselhaus, there are bound to be certain papers that might get a bit lost in the corridors of the mind—papers that make only moderate strides, perhaps, or that involve relatively little effort or input (when, for example, being a minor consulting author on a paper with many coauthors). Conversely, there are always standout papers that one can never forget—for their scientific impact, for coinciding with particularly memorable periods of one’s career, or for simply being unique or beastly experiments.
Millie’s first major research publication after becoming a permanent member of the MIT faculty fell into the standout category. It was one she described time and again in recollections of her career, noting it as “an interesting story for history of science.”
The story begins with a collaboration between Millie and Iranian American physicist Ali Javan. Born in Iran to Azerbaijani parents, Javan was a talented scientist and award-winning engineer who had become well known for his invention of the gas laser. His helium-neon laser, coinvented with William Bennett Jr. when both were at Bell Labs, was an advance that made possible many of the late twentieth century’s most important technologies—from CD and DVD players to bar-code scanning systems to modern fiber optics.
After publishing a couple of papers describing her early magneto-optics research on the electronic structure of graphite, Millie was looking to delve even deeper, and Javan wanted to help. The two met during Millie’s work at Lincoln Lab; she was a huge fan, once calling him “a genius” and “an extremely creative and brilliant scientist.”
For her new work, Millie aimed to study the magnetic energy levels in graphite’s valence and conduction bands. To do this, she, Javan, and a graduate student, Paul Schroeder, employed a neon gas laser, which would provide a sharp point of light to probe their graphite samples. The laser had to be built especially for the experiment, and it took years for the fruits of their labor to mature; indeed, Millie moved from Lincoln to MIT in the middle of the work.
If the experiment had yielded only humdrum results, in line with everything the team had already known, it still would have been a path-breaking exercise because it was one of the first in which scientists used a laser to study the behavior of electrons in a magnetic field. But the results were not humdrum at all. Three years after Millie and her collaborators began their experiment, they discovered their data were telling them something that seemed impossible: the energy level spacing within graphite’s valence and conduction bands were totally off from what they expected. As Millie explained to a rapt audience at MIT two decades later, this meant that “the band structure that everybody had been using up till that point could certainly not be right, and had to be turned upside down.”
In other words, Millie and her colleagues were about to overturn a well-established scientific rule—one of the more exciting and important types of scientific discoveries one can make. Just like the landmark 1957 publication led by Chien-Shiung Wu, who overturned a long-accepted particle physics concept known as conservation of parity, upending established science requires a high degree of precision—and confidence in one’s results. Millie and her team had both.
What their data suggested was that the previously accepted placement of entities known as charge carriers within graphite’s electronic structure was actually backward. Charge carriers, which allow energy to flow through a conducting material such as graphite, are essentially just what their name suggests: something that can carry an electric charge. They are also critical for the functioning of electronic devices powered by a flow of energy.
Electrons are a well-known charge carrier; these subatomic bits carry a negative charge as they move around. Another type of charge carrier can be seen when an electron moves from one atom to another within a crystal lattice, creating something of an empty space that also carries a charge—one that’s equal in magnitude to the electron but opposite in charge. In what is essentially a lack of electrons, these positive charge carriers are known as holes.
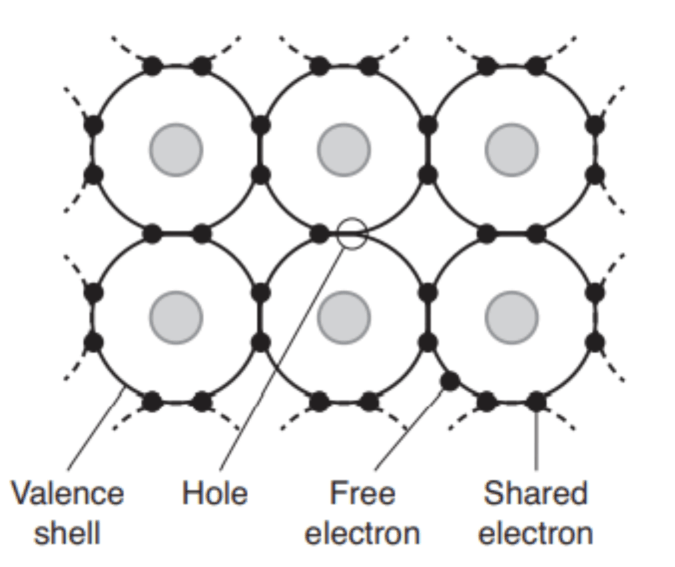
MIT Press
FIGURE 6.1 In this simplified diagram, electrons (black dots) surround atomic nuclei in a crystal lattice. In some circumstances, electrons can break free from the lattice, leaving an empty spot or hole with a positive charge. Both electrons and holes can move about, affecting electrical conduction within the material.
Millie, Javan, and Schroeder discovered that scientists were using the wrong assignment of holes and electrons within the previously accepted structure of graphite: they found electrons where holes should be and vice versa. “This was pretty crazy,” Millie stated in a 2001 oral history interview. “We found that everything that had been done on the electronic structure of graphite up until that point was reversed.”
As with many other discoveries overturning conventional wisdom, acceptance of the revelation was not immediate. First, the journal to which Millie and her collaborators submitted their paper originally refused to publish it. In retelling the story, Millie often noted that one of the referees, her friend and colleague Joel McClure, privately revealed himself as a reviewer in hopes of convincing her that she was embarrassingly off-base. “He said,” Millie recalled in a 2001 interview, “‘Millie, you don’t want to publish this. We know where the electrons and holes are; how could you say that they’re backwards?’” But like all good scientists, Millie and her colleagues had checked and rechecked their results numerous times and were confident in their accuracy. And so, Millie thanked McClure and told him they were convinced they were right. “We wanted to publish, and we… would take the risk of ruining our careers,” Millie recounted in 1987.
Giving their colleagues the benefit of the doubt, McClure and the other peer reviewers approved publication of the paper despite conclusions that flew in the face of graphite’s established structure. Then a funny thing happened: bolstered by seeing these conclusions in print, other researchers emerged with previously collected data that made sense only in light of a reversed assignment of electrons and holes. “There was a whole flood of publications that supported our discovery that couldn’t be explained before,” Millie said in 2001.
Today, those who study the electronic structure of graphite do so with the understanding of charge carrier placement gleaned by Millie, Ali Javan, and Paul Schroeder (who ended up with quite a remarkable thesis based on the group’s results). For Millie, who published the work in her first year on the MIT faculty, the experiment quickly solidified her standing as an exceptional Institute researcher. While many of her most noteworthy contributions to science were yet to come, this early discovery was one she would remain proud of for the rest of her life.
All products recommended by Engadget are selected by our editorial team, independent of our parent company. Some of our stories include affiliate links. If you buy something through one of these links, we may earn an affiliate commission.

